Jahanpour made the remarks at the 24th session of the Legal Commission for Diagnosis (the competence to manufacture and enter medicines and biological substances) of the Food and Drug Administration.
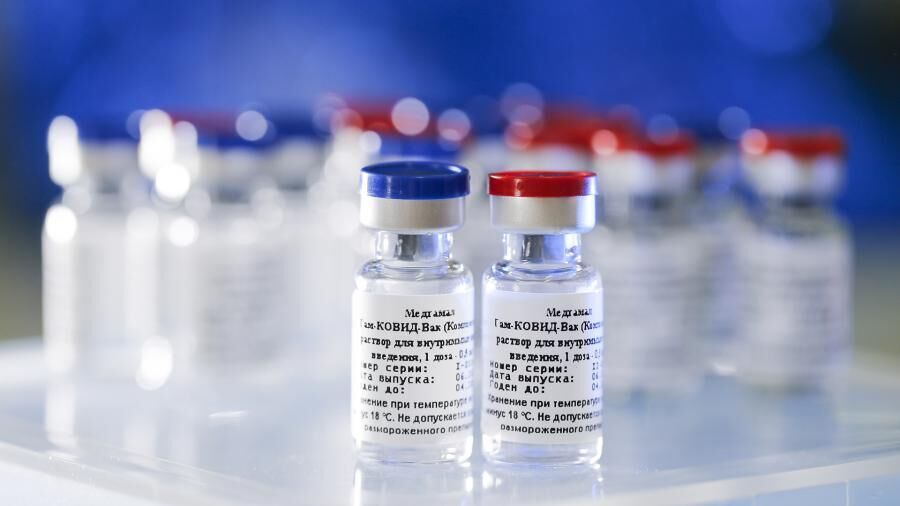
He said the committee has given emergency use authorization (EUA) for the Sputnik V vaccine made by the Gamaleya Center of Russia in the form of the freeze-dried solution as well as single-dose and five-dose vials.
Jahanpour noted that the Food and Drug Administration is evaluating other vaccines made in India, China, and Russia.
Earlier, speaking to IRNA, he said as the Minister of Health had previously stated and the President had emphasized, there are four different ways to supply the coronavirus vaccine, including direct purchase from a foreign country, procurement from the World Health Organization’s COVAX facility, a joint production with a Cuban company as well as domestic production of the vaccine.
The arrangements for the joint production of the vaccine with a foreign country have been pursued. He noted that Iran set conditions for conducting a human clinical trial of the vaccine which is that it should be jointly produced and its technology should be transferred to Iran, he added.
One of the well-known institutions in Cuba, which has old cooperation with the Pasteur Institute of Iran in the field of vaccine production for many years, accepted to kick off the joint venture, he noted.
Cuba has completed phase one of the human clinical trial of the coronavirus vaccine, the second phase of the human clinical study is currently being carried out under the supervision of the Pasteur Institute of Iran in the Latin American country, he said, adding that it will be continued in Iran within the coming months.
3266**2050
Follow us on Twitter @IrnaEnglish


Your Comment